Some Known Details About Pharmacological interventions for smoking cessation - North
Not known Incorrect Statements About Smoking Cessation: Less Expensive Cytisine May Be As
A generic drug extensively utilized in Eastern European and Asian countries for cigarette smoking cessation handled the West's leading non-nicotine agent in a randomized trial, coming out on the brief end, scientists said. cytisine smoking cessation review for 25 days stopped working to meet requirements for noninferiority in comparison with varenicline (Chantix) given for 84 days in an open-label trial involving 1,452 smokers intending to quit the habit, reported Ryan J.
The finding was a significant dissatisfaction because cytisine-- a plant alkaloid that, like varenicline, stimulates nicotinic acetylcholine receptors-- had previously been revealed to be exceptional to placebo and to basic nicotine replacement treatment (NRT) in different trials. Additionally, a trial including some of the very same scientists and reported previously this year, conducted amongst native Maori and member of the family in New Zealand, found that cytisine was more effective than varenicline.
Extended dosing would deserve testing in a future study, they indicated. And the contrary results in the Maori trial might suggest that populations more accepting of "natural" products would respond better to cytisine than to varenicline. A few of these questions might be responded to in an continuous, placebo-controlled, phase III trial with a proprietary cytisine solution called cytisinicline, in which the agent is given for up to 12 weeks.
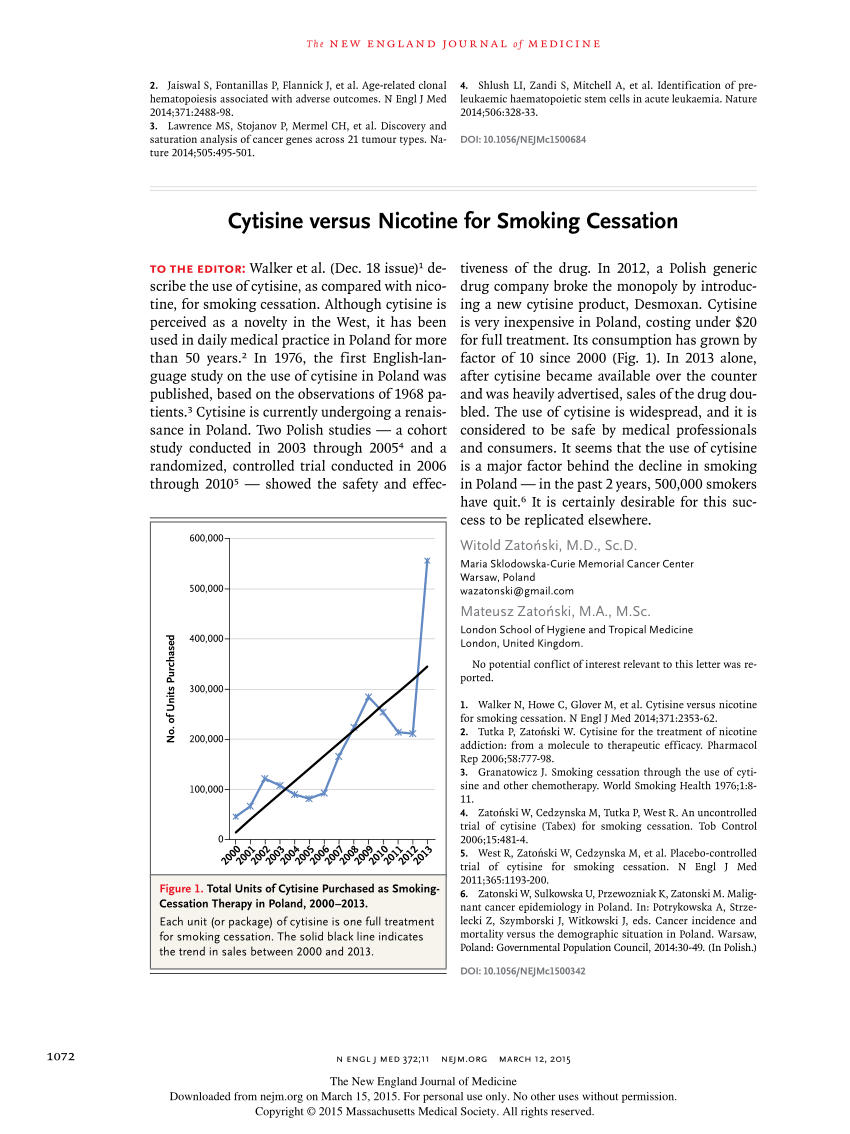 Medications Are Effective to Help People Quit Smoking, Research Finds - TIME.com
Medications Are Effective to Help People Quit Smoking, Research Finds - TIME.comAs a partial agonist for nicotinic acetylcholine receptors, it reportedly reduces nicotine yearnings and withdrawal symptoms when people stop smoking cigarettes. The standard treatment interval has actually been 25 to thirty days, although Courtney and colleagues noted that this isn't necessarily optimum-- as an inexpensive plant derivative, it hasn't had the monetary backing to test several dosing routines as Huge Pharma would provide for an item that needs FDA approval.
 PDF] The effectiveness, safety and cost-effectiveness of cytisine versus varenicline for smoking cessation in an Australian population: a study protocol for a randomized controlled non-inferiority trial- Semantic Scholar
PDF] The effectiveness, safety and cost-effectiveness of cytisine versus varenicline for smoking cessation in an Australian population: a study protocol for a randomized controlled non-inferiority trial- Semantic Scholar Medications Are Effective to Help People Quit Smoking, Research Finds - TIME.com
Medications Are Effective to Help People Quit Smoking, Research Finds - TIME.comSome Known Factual Statements About Cytisine for smoking cessation: as good as nicotine
It's not without debate, naturally-- early reports of psychiatric disturbances including suicidality caused label warnings, although the FDA still considers it a safe and efficient drug. Then simply last week, drugmaker Pfizer recalled nine lots of varenicline (which had not yet been delivered to drug stores) because of possible nitrosamine contamination.
Nevertheless, varenicline has actually been the leading non-NRT drug for cigarette smoking cessation in the the Western world. For cytisine to stake a claim as a reliable agent-- particularly in nations aside from the U.S. that would want proof of a minimum of noninferiority for it to be consisted of in nationwide formularies-- a head-to-head trial in a Western-type population might assist its case.

